Society for Gynecologic Oncology (SGO) 2018 – STRO-002 Abstract
Preclinical Activity and Safety of STRO-002, a novel ADC targeting Folate Receptor Alpha for Ovarian Cancer
Objectives: Folate receptor alpha (FolRa) is a cell-surface glycoprotein, highly expressed in ovarian and endometrial adenocarcinoma, that is a promising target for cancer therapy using antibody drug conjugates (ADCs). We have employed an E. coli cell-free expression system (Xpress CF+™) and site-specific conjugation technology to generate STRO-002, a novel, site-specific, and homogenous FolRa-targeting ADC.
Methods: Wehave conducted preclinical studies demonstrating anti-tumor activity, optimal ADC stability, and favorable safety profile of STRO-002. In vitro cytotoxicity assays and in vivo efficacy studies were conducted to evaluate the activity of STRO-002 in multiple ovarian cancer cell lines and xenografts. Exploratory toxicology studies were conducted to determine the safety profiles for STRO-002 and its metabolite SC209 in cynomolgous monkeys and rats, respectively.
Results: Potent in vitro cytotoxicity (EC50= 0.1-3 nM) was observed in multiple ovarian cancer cell lines treated with STRO-002. STRO-002 exhibits dose-dependent tumor growth inhibition in Igrov-1 tumor xenografts at a single dose (Figure 1) and complete regression is achieved in Igrov-1 and OVCAR-3 tumors with a single dose at 10 and 5 mg/kg, respectively. In addition, administration of STRO-002 in combination with carboplatin confers added benefit in efficacy in Igrov-1 tumors. Exploratory toxicology studies show favorable safety profiles for STRO-002 and SC209. The main toxicity finding in monkeys dosed up to 10 mg/kg was reversible hematopoietic/lymphoid tissue toxicity, which is considered antigen-independent and consistent with the anti-proliferative effects of SC209 observed in single-dose toxicology studies in rats. No signs of ocular toxicity were observed. Additionally, the drug-linkage in STRO-002 is highly stable in circulation and the released warhead, SC209, is a very weak substrate for cellular drug-resistance efflux pumps and is cleared rapidly from plasma.
Conclusions: STRO-002 is an ADC with minimal drug moiety release in circulation and the potential for an improved safety and activity profile, and areduced risk of tumor drug resistance.These data support advancing STRO-002 to IND-enabling studies as a potential treatment of FolRaexpressing malignancies such as ovarian and endometrial cancer.
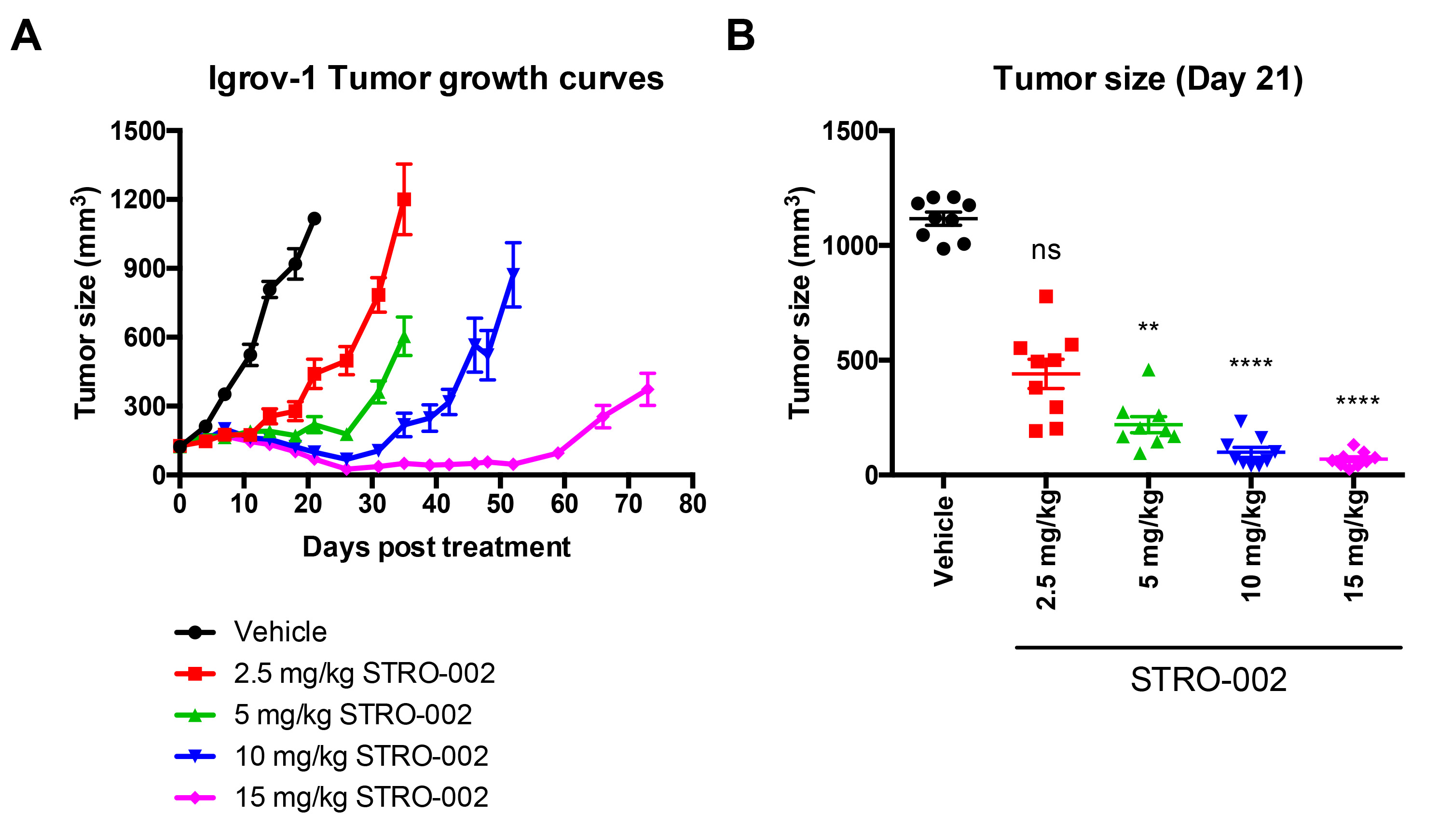
Figure 1: Dose Dependent Effect of STRO-002 on Igrov-1 Tumor Growth
