
A registrational directed study of luveltamab tazevibulin that is redefining FRα positivity in platinum-resistant ovarian cancer¹´²
FRα TPS* 25% or Higher
Irrespective of Staining Intensity
*Tumor Proportion Score
Lowering the FRα threshold to 25% expands the definition of actionable FRα expression, extending the opportunity for targeted therapy to more women with platinum-resistant ovarian cancer

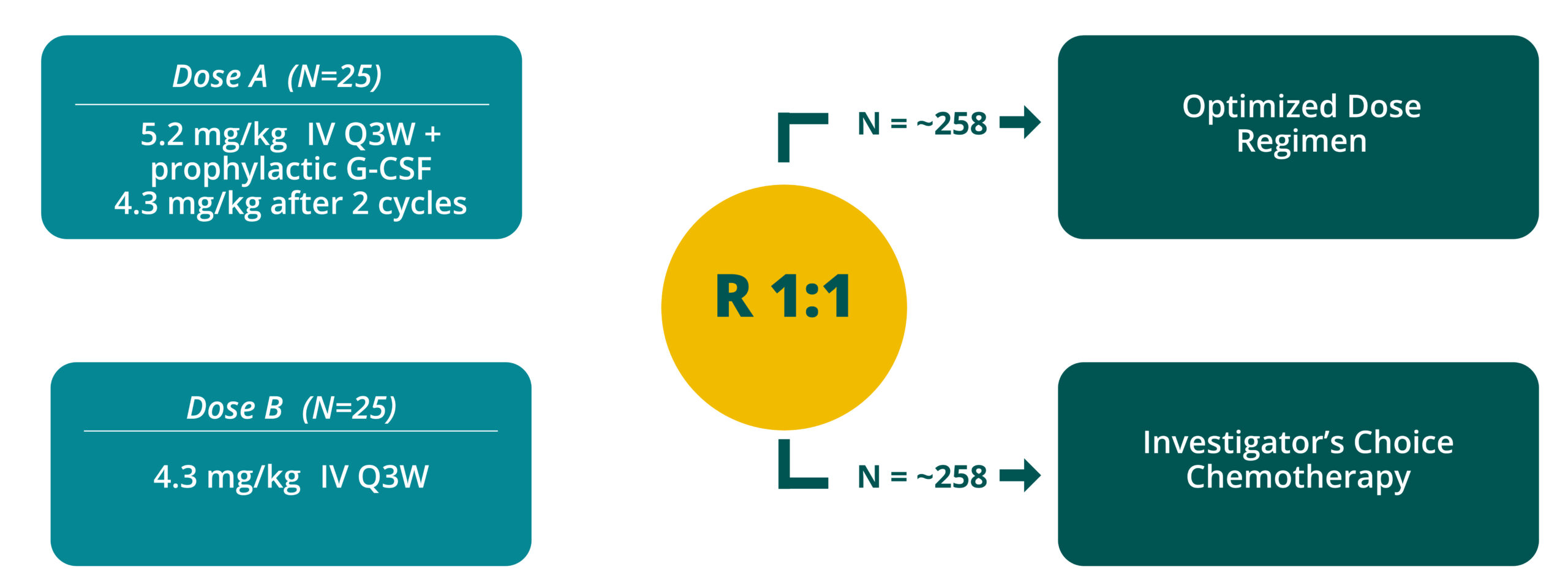
Q3W = every 3 weeks
Global study in 20+ countries
in collaboration with ENGOT, GOG, and APGOT
REFRαME-O1 other study ID numbers:
STRO-002-GM3, ENGOT-OV79, GOG-3086, GEICO-134-O, APGOT-OV9

of platinum-resistant ovarian cancer patients are potentially eligible for REFRαME-O1
Additional Trial Information

Email Sutro at REFRaME@sutrobio.com to learn more
Sutro Biopharma is a clinical-stage ADC company relentlessly focused on the discovery and development of precisely designed cancer therapeutics, transforming what science can do for patients
References: 1. Data on file. Sutro Biopharma, Inc. 2. Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41(13):2436-2445.
